
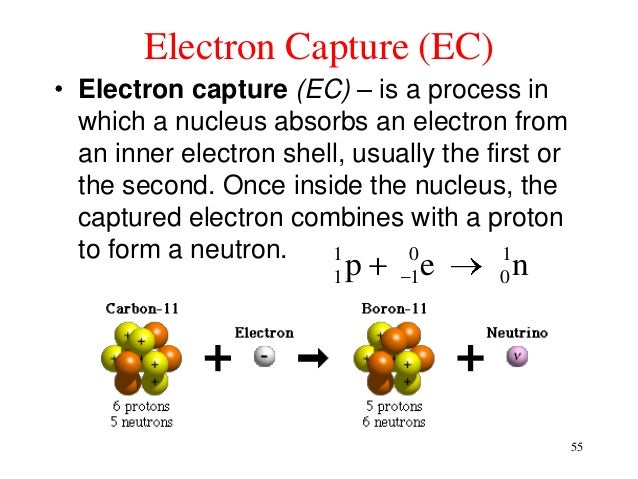
This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino.Į or when written as a nuclear reaction equation, e − 1 0 + p 1 1 ⟶ n 0 1 + 0 0

The outer electron is ejected from the atom, leaving a positive ion.Įlectron capture ( K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Lower right: In the Auger effect, the energy absorbed when the outer electron replaces the inner electron is transferred to an outer electron. An x-ray, equal in energy to the difference between the two electron shells, is emitted.
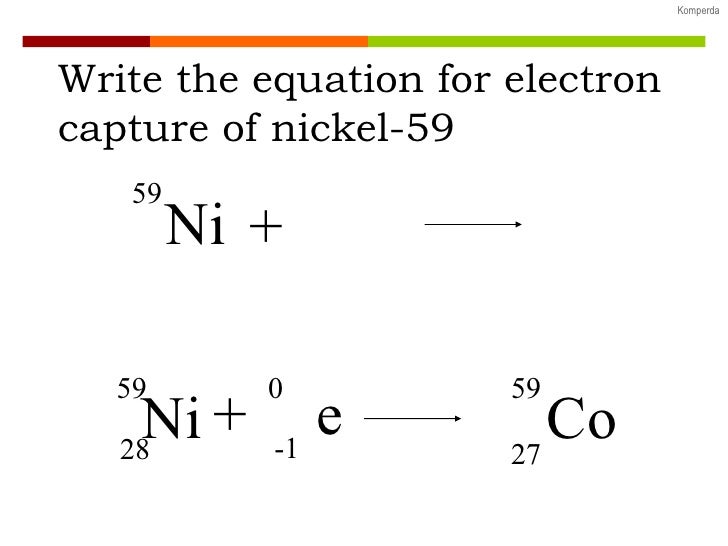
Lower left: An outer electron replaces the "missing" electron.


 0 kommentar(er)
0 kommentar(er)
